JUMP TO TOPIC
Degree of Unsaturation Calculator + Online Solver With Free Steps
The Degree of Unsaturation Calculator is used to calculate the Index of Hydrogen Deficiency IHD or the degrees of unsaturation of different hydrocarbons. It also provides structures of various isomers for the input hydrocarbon.
Unsaturation of a compound means having the tendency to dissolve in a solution. The compound with a greater degree of unsaturation has a higher capability to dissolve in a solution so the calculator measures the dissolving capability of a substance in a solution.
A saturated compound means that the compound is dissolved or absorbed in the mixture to the highest extent and cannot be further dissolved. The degree of unsaturation varies with different types of hydrocarbons. Alkanes are hydrocarbons with single carbon-carbon bonds.
The carbon atoms are filled to the maximum number of hydrogen atoms in alkanes thus having the least degree of unsaturation. The IHD for alkanes is 0 as they are fully saturated.
Alkenes are open-chain hydrocarbons containing double bonds between carbon atoms. The presence of a double bond makes the compounds unsaturated. For every double bond in the compound, there is a hydrogen deficiency.
So the degree of unsaturation for alkenes is 1 if one double bond is present between the carbon atoms.
Alkynes are hydrocarbons containing triple carbon-carbon bonds. A triple bond makes the compound more hydrogen deficient. For every triple bond, the value of IHD is 2. Alkynes are the most unsaturated hydrocarbons.
Closed chain hydrocarbons form a ring of carbon atoms. The degree of unsaturation of a ring of carbon atoms is 1 same as having a double bond in the case of alkenes.
What Is a Degree of Unsaturation Calculator?
The Degree of Unsaturation Calculator is an online tool that is used to calculate the degree of unsaturation of hydrocarbons and also displays the structures of isomers of the hydrocarbon.
The degree of unsaturation talks about the molecular structure of the compound. The same compound can be a ketone or alcohol depending upon the degrees of unsaturation.
The formula for Degree of Unsaturation used by the calculator is:
\[ DoU = \frac{ 2C + 2 \ – \ H }{ 2 } \]
Where C and H represent the number of carbon atoms and hydrogen atoms in the compound respectively.
How To Use the Degree of Unsaturation Calculator
The user can follow the steps given below to use the Degree of Unsaturation Calculator.
Step 1
The user must first enter the molecular formula of the hydrocarbon for which the degree of unsaturation is required.
It should be entered in the block titled, “Enter the Molecular Formula:” in the input tab of the calculator.
For the default example, the molecular formula used is $ C_3 H_4 $.
Step 2
After entering the molecular formula, the user must now press the button, “Calculate Degree of Unsaturation” for the calculator to process the input.
Output
The calculator computes the Degree of Unsaturation and displays the output in the three windows given below.
Input Interpretation
The calculator interprets the input and displays the molecular formula in this window. For the default example, it displays the formula given below.
$C_3 H_4$ = degrees of unsaturation
Result
The calculator displays the Degree of Unsaturation (DoU) or the Index of Hydrogen Deficiency (IHD) in this window.
For the default example, the number of carbon atoms is 3 and the number of hydrogen atoms is 4. Putting the values of C and H in the DoU gives:
\[ DoU = \frac{ 2(3) + 2 \ – \ 4 }{ 2 } \]
\[ DoU = \frac{ 6 \ – \ 2 }{ 2 } \]
\[ DoU = \frac{ 4 }{ 2 } \]
The calculator displays the result as follows:
DoU = 2
Isomers
Isomers are compounds having the same molecular formula but different molecular structures. DoU helps in determining the particular isomer required by the user.
For the default example, the isomers for $ C_3 H_4 $ are as follows:
The structure of methyl acetylene is shown in figure 1.
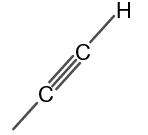
Figure 1
The structure of propadiene is shown in figure 2.
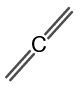
Figure 2
The structure of cyclopropene is shown in figure 3.
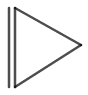
Figure 3
Solved Examples
Following are some of the examples solved through the Degree of Unsaturation Calculator.
Example 1
For the hydrocarbon $ C_5 H_{12} $ , find the degrees of saturation and also draw the molecular structures of different isomers of the compound.
Solution
The user must first enter the molecular formula $ C_5 H_{12} $ in the input tab of the calculator. After pressing “Calculate Degree of Unsaturation”, the calculator displays the output as follows.
The Input Interpretation window shows the molecular formula as given below:
$C_5 H_{12}$ = degrees of unsaturation
The calculator computes the degree of unsaturation DoU for $ C_5 H_{12} $ and shows the result as follows:
DoU = 0
The calculator also displays the isomers of $ C_5 H_{12} $ which are N-pentane, 2,2-dimethylpropane, and isopentane.
The molecular structure for N-pentane is shown in figure 4.

Figure 4
The structure for 2,2-dimethylpropane is shown in figure 5.

Figure 5
The molecular structure for isopentane is shown in figure 6.
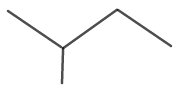
Figure 6
Example 2
Calculate the degree of unsaturation or the Index of hydrogen deficiency for the compound $ C_4 H_{10} $. Also, draw the different molecular structures for this hydrocarbon.
Solution
The chemical formula $ C_4 H_{10} $ should be entered in the input tab of the calculator. The user must now press, “Calculate Degree of Unsaturation” for the calculator to compute the degree of unsaturation.
The calculator interprets the input and shows the entered molecular formula as given below:
$C_4 H_{10}$ = degrees of unsaturation
The calculator computes the degree of unsaturation and shows the result as follows:
DoU = 0
The calculator also displays the isomers for $ C_4 H_{10} $ which are butane and isobutane.
The structure for butane is shown in figure 7.

Figure 7
The molecular structure for isobutane is shown in figure 8.
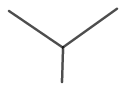
Figure 8
All the images are created using Geogebra.
