JUMP TO TOPIC
Empirical Formula Calculator + Online Solver With Free Steps
The online Empirical Formula Calculator is a free tool that helps you find the Empirical Formula of any given chemical composition. The input of the Empirical Formula Calculator is the name and percentage mass of elements. The result is the simplest whole number ratio of atoms in the given compound, known as the Empirical Formula.
What Is the Empirical Formula Calculator?
The Empirical Formula Calculator is an online calculator that is used to find the empirical formula on compounds.The Empirical formula is widely used by chemists for multiple purposes, hence this online empirical formula calculator is very useful for them. When you solve problems regarding empirical formulas, you need to do many calculations and approximations, therefore you need a tool that can solve the problems quickly irrespective of the complexity of given compounds.The Empirical Formula calculator directly gives you the results in a few seconds. One interesting feature of this tool is that it also gives a Pie Chart which represents the mass composition of the compound.How To Use the Empirical Formula Calculator?
You can use the Empirical Formula Calculator by entering the name of the element and the percentage of its composition in the compound. The procedure for using the Empirical Formula calculator is user-friendly.Step 1
You just need to enter the data in the following pattern:Percentage composition – first Element Name, Percentage Composition – Second Element name, Third Element Name, Percentage Composition, and so on. For example, 10% carbon, 30% oxygen, and 5% hydrogen of a compound.This calculator is easily accessible as compared to those which you first have to download and install. It gives quick results which save time and avoid tedious handwriting efforts. Also, the accurate results are displayed via pie charts.Step 2
Click on the “submit” button to obtain the results.Results
Now you can see the results in a new window which includes an empirical formula. You can see the pie chart by clicking on the pie chart button.How Does the Empirical Formula Calculator Work?
The empirical formula calculator works by finding the ratio of the number of moles of atoms of the compound.The following steps are followed by the Empirical Formula Calculator to find the desired output:Find the Number of Moles
In this step, the Empirical Formula Calculator determines the moles of each element present in the compound. The following formula is used to find the number of moles:Number of moles = mass given in grams / Molar Mass of the element
The molar mass of each element depends on the isotopes of the elementsLet’s understand what’s meant by molar mass and moles of a chemical compound.Molar Mass
The mass of one mole of the chemical compound in grams is known as the Molar Mass of a substance. The standard unit of Molar Mass is $gmol^{-1}$Mole
Mole is the standard unit used to measure the amount of substance in chemistry. Whereas substance here means anything that has some mass and occupies space.The number of atoms present in one mole of a substance is known as Avogadro’s Number. Its calculated value is $6.022 \times 10^{23}$.Find the Ratio of Moles
Now, divide the number of moles of each element by the smallest number of moles that were found in the previous step. This gives the simplest formula.Result
If the number of moles got in the previous step are all whole or very close to the whole numbers, the empirical formula will be written with the whole number as a subscript of each element.In case, all the moles are not in the whole number, the empirical formula calculator multiplies each of the moles by the smallest whole number that converts each decimal number into a whole number. This gives the empirical formula of the given compound.Pie-Chart
The Empirical Formula calculator also shows a pie chart of the results. This pie chart is a graph that represents the percentage of elements in the compound in a circular graph.The slices or parts of the pie chart represent the relative amount/mass of elements in a chemical compound. Different colors in the pie chart represent different elements of the compound under consideration.What Is the Empirical Formula?
The simplest positive integer ratio of each type of atom present in a compound is known as the Empirical Formula of a chemical compound.In simple words, the concept of Empirical Formula can be understood with the example of sulfur monoxide. Its empirical formula would be SO because it is derived from the formula $S_{2}O_{2}$.Empirical Formulas may represent several different chemical structures, they are not unique like molecular formulas.To determine the amount or percent of a particular element of which the sample is composed, specific elemental analysis tests are conducted. This percentage is then utilized in finding the empirical formulas of compounds.What Is Molecular Formula?
The actual number of atoms of each element in a molecule of a compound is known as the molecular formula of a compound.The molecular Formula can be calculated with the ratio of molar mass and mass of the empirical formula.The molecular formula and the empirical formula are usually the same, otherwise, it is an exact multiple of the empirical formula.Advantages and Limitations of the Empirical Formula
Let’s discuss some of the advantages of the empirical formula of a compound along with some of its limitations.Advantages
Empirical Formulas are important because it reveals the relative amount of all the elements in a molecule which is very useful for determining the molecular formula of a compound.The Empirical Formula of elements is very beneficial in experimental settings as it is a basic factor for calculating the molecular formula of the compounds.Similarly, the empirical formula helps chemists to know how reactive a chemical may be.Limitations
The empirical formula on a compound is limited to the ratio of atoms of the compound and lacks giving the actual number of atoms present in that compound. The true identity of the compound is not given by the empirical formula.Solved Examples
Now let’s solve some examples to understand the working of the Empirical Formula calculator. The examples are described below:Example 1
A molecule contains 32.65% Sulphur, 2.04% hydrogen, and 65.3% Oxygen. Find the empirical formula of this compound.Solution
Firstly, it changes the percent to grams.32.65 % equals 32.65 grams of Sulphur
65.3 % equals 65.3 grams of Oxygen
2.04 % equals 2.04 grams of Hydrogen
Now, it divides all the given masses by their molar mass.32.65 g of Sulphur/32 $gm^{-1}$ = 1.0203 moles of Sulphur
65.3 g of Oxygen/16 $gm^{-1}$ = 4.08 moles of Oxygen
2.04 g of Hydrogen/1.008 $gm^{-1}$ = 2.024 moles of Hydrogen
Further, it takes the smallest number of moles from the previous step and divides all the answers by this number. Also, it rounds off the decimal point to the nearest whole number.Then, picks the smallest answer in moles from the previous step and divide all the answers by that. In this case, 1.0203 is the smallest number so,1.0203 moles of S/1.0203 = 1
4.08 moles of O/1.0203 = 3.998 $\approx$ 4
2.024 moles of H/1.0203 = 1.984 $\approx$ 2
Finally, the values obtained in the previous step become the subscripts in the formula of the respective chemical.S = 1, O = 4, H = 2
The empirical formula of the given chemical compound is:\[H_{2}SO_{4} \]Pie-Chart
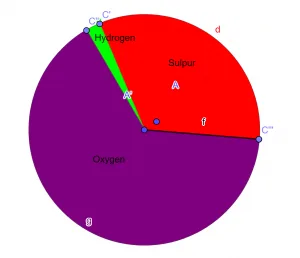
Figure 1
Example 2
A chemical compound was analyzed in the laboratory which should that this chemical contains 76% fluorine atoms and 24% carbon atoms by mass. As a chemistry student derive the Empirical Formula for this compound.Solution
Grams of Fluorine = 76 grams
Grams of Carbon = 24 grams
Moles of Flourine = 76 grams/19 grams per mole = 4 moles
Moles of Carbon = 24 grams/12.01 grams per mole = 2 moles
As 2 moles is the smallest number, so the Empirical Formula Calculator divides all atoms by 2.\[ Fluorine = \frac{4}{2} = 2 \]\[ Carbon = \frac{2}{2} = 1 \]Result
So, the empirical formula for the given compound is $CF_{2}$.Pie-Chart
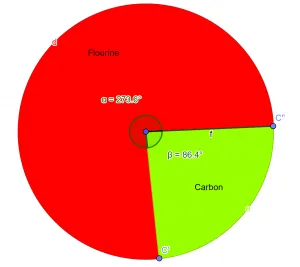
Figure 2
Example 3
A chemical compound is composed of 88.79% oxygen atoms and 11.19% hydrogen atoms. Determine the empirical formula for this chemical compound.Solution
Grams of Oxygen per 100 grams of compound = 88.79 grams
Grams of Hydrogen per 100 grams of compound = 11.19 grams
Now, the empirical formula calculator converts grams of each element to molesMoles of Oxygen = 88.79 g / 16 g per mole oxygen = 5.549 moles oxygen atoms
Moles of Hydrogen = 11.19 g / 1.009 g per mole hydrogen = 11.10 moles of hydrogen atoms
Now it divides the results with the lowest moles of atoms in the previous step.So, it givesHydrogen = 2, Oxygen = 1
Result
The empirical formula of the given compound is $H_{2}O$Pie-Chart
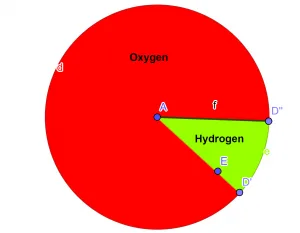
Figure 3
