JUMP TO TOPIC
Percent Composition Calculator + Online Solver With Free Steps
The online Percent Composition Calculator is a calculator that finds the percentage composition of a chemical compound.
The Percent Composition Calculator is an easy-to-use calculator that helps chemists and scientists quickly determine the percentage composition of a chemical compound.
What Is a Percent Composition Calculator?
A Percent Composition Calculator is an online calculator that allows you to determine the percentage composition of a chemical compound.
The Percent Composition Calculator takes only a single input: the compound’s chemical formula. The calculator then displays a range of details about the percentage composition of the compound.
How To Use the Percent Composition Calculator?
To use the Percent Composition Calculator, you input the compound’s chemical formula into the calculator and click the “Submit” button.
The detailed instructions for using the Percent Composition Calculator are given below:
Step 1
To use the Percent Composition Calculator, you need first enter the compound’s chemical formula in its respective box.
Step 2
After entering the compound’s chemical formula into the Percent Composition Calculator, you click the “Submit” button. The calculator will open a new window and display the chemical compound’s detailed properties and percentage composition.
How Does a Percent Composition Calculator Work?
The Percent Composition Calculator works by taking the compound’s chemical formula and a ratio between the elements in the mixture. The ratio is then multiplied by 100 to give us a percentage.
The following formula can be used to calculate the percentage composition of a compound:
\[ \% C_{E}= \frac{g^{E}}{g^{T}} \times 100 \]
What Is a Percent Composition?
The percent composition of any given compound is the ratio of the quantity of each element contained in the compound to the total amount of individual components that are present in the compound multiplied by 100. In this case, the quantity is expressed in grams of the elements contained in the solution.
The percent composition of any chemical is an expression of its composition in terms of all the elements present. The chemical analysis reveals the importance of this composition calculation.
The following formula can represent the percentage composition:
\[ \% C_{E}= \frac{g^{E}}{g^{T}} \times 100 \]
The percent composition of an element E is represented by percent CE. This is the value we will compute. The numerator on the right side represents the total quantity of element E present in the compound. The denominator, however, represents the total amount of all the elements in the mixture.
What Is Mass Percent Composition?
The mass percent composition represents the concentration of an element in a compound or a component in a combination. This word refers to the overall percentage by mass of each element present in a compound.
It is vital to remember that the mass percent composition can be calculated by dividing the mass of a component by the total mass of the combination. This proportion is then multiplied by 100. It is also known as the mass percent $(\frac{w}{w})\%$.
This formula is useful for determining the smallest entire number of moles and the relative number of atoms in each element of a compound. Chemists can derive the real molecular formula using the empirical formula. This formula specifies the number of atoms in the chemical.
Solved Examples
The Percent Composition Calculator instantly allows you to find the percentage composition of a chemical compound.
The following examples are solved using the Percent Composition Calculator:
Example 1
While experimenting, a chemist comes across the following strong acid:
\[ H_{2}SO_{4} \]
The chemist needs to find the percentage composition of the strong acid to complete his experiment. Use the Percent Composition Calculator to find the percent composition of the strong acid.
Solution
Using the Percent Composition Calculator, you can easily determine the percentage composition of the chemical compound. First, we input the compound’s chemical formula into the Percent Composition Calculator; the compound’s chemical formula is $H_{2}SO_{4}$.
Once we have input the compound’s chemical formula into the Percent Composition Calculator, we click the “Submit” button. The calculator quickly finds the percentage composition of the compound along with its properties in a new window.
The following results are taken from the Percent Composition Calculator:
Input Interpretation
Sulphuric Acid
Chemical Names and Formulas
\[ \text{Formula} = H_{2}SO_{4} \]
\[ \text{Hill \ Formula} = H_{2}O_{4}S \]
Name = Sulphuric Acid
Structure Diagram
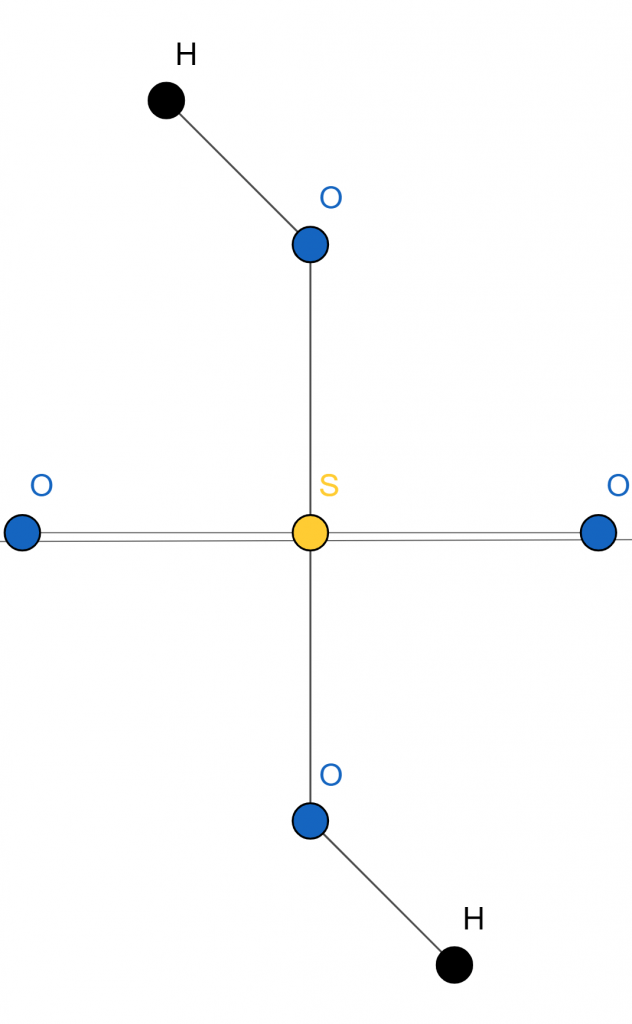
Figure 1
Properties
Here are some basic properties of sulphuric acid determined using this calculator:
Molar mass = 98.07 g/mol
Phase = liquid (at STP)
Melting Point = $10.371^{\circ}$C
Boiling Point = $279.6^{\circ}$C
Density = 1.8305 g/$cm^{3}$
Solubility in water = very soluble
Liquid Properties (at STP)
Here are the liquid properties of Sulphuric acid at standard temperature and pressure:
Density = 1.8305 g/$cm^{3}$
Vapor Pressure = 6 x $10^{-5}$ mmHg
Dynamic Viscosity = 0.021 Pas (at $25^{\circ}$C)
Surface Tension = 0.0735 N/m
Refractive Index = 1.41827
Thermodynamic Properties
Thermodynamic properties are given below:
Specific heat capacity cp (liquid) = 1.416 J/(gK)
Specific free energy of formation $\Delta_{f}G^{\circ}$ (liquid) = -7.036 kJ/g
Specific heat of formation $\Delta_{f} H^{ \circ}$ (aqueous) = -9.269 kJ/g
Specific heat of formation $\Delta_{f} H^{ \circ}$ (liquid) = -8.3 kJ/g}
Specific entropy $S^{\circ}$ (aqueous) = 0.2039 J/(gK)
Specific entropy $S^{\circ}$ (liquid) = 1.601 J/(gK)
Specific heat of vaporization = 0.57 KJ/J
Specific heat of fusion = 0.1092 KJ/g
Chemical Identifiers
Every chemical has certain associated numbers. For Sulphuric acid various identifiers are listed below:
CAS number = 7664-93-9
Beilstein number = 2037554
PubChem CID Number = 1118
PubChem SID Number = 24859176
SMILES identifier = OS(=O)(=O)O
Toxicity Property
The following properties identify the smell and odor of the chemical substance:
Odor = odorless
Threshold limit value = 0.05 ppmv
Ion Equivalent
The ionic composition is given below:
$H^{+}$ (hydrogen cation) = 2
$SO_{4}^{2-}$ (sulfate anion) = 1
Example 2
A scientist needs to find the percentage composition of hydrochloric acid HCl. Using the Percent Composition Calculator, find the percent composition of hydrochloric acid.
Solution
You can simply determine the percentage composition of a chemical substance using the Percent Composition Calculator. First, we enter the compound’s chemical formula into the Percent Composition Calculator; the compound’s chemical formula is HCl.
We click the “Submit” button after entering the compound’s chemical formula into the Percent Composition Calculator. The calculator instantly determines the compound’s percentage composition and attributes in a new window.
The following results are extracted from the Percent Composition Calculator:
Input Interpretation
The input entered in the calculator is interpreted in the following manner:
Hydrogen chloride
Chemical Name and Formula
The chemical name and formula of the acid are given below:
Formula = HCl
Name = hydrogen chloride
Structure Diagram
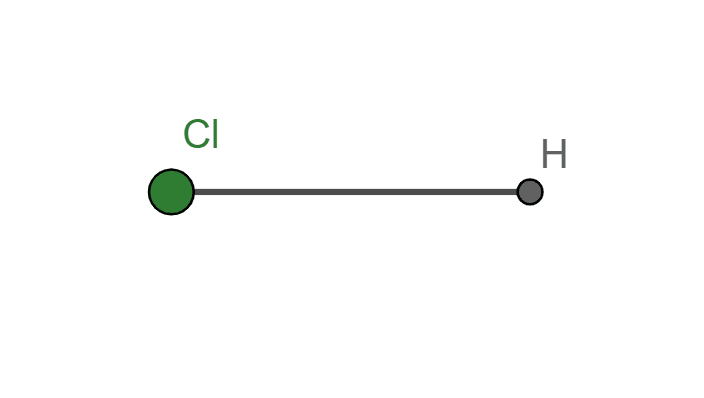
Figure 2
Basic Properties
Molar mass = 36.46 g/mol
Phase = gas (at STP)
Melting Point = $-114.17^{\circ}C$
Boiling Point = $-85^{\circ}C$
Density = 0.00149 g/$cm^{3}$ (at $25^{\circ}$C
Solubility in water = miscible
Gas Properties (at STP)
Gaseous properties of HCL at standard pressure and temperature conditions are given below:
Density = 0.00149 g/$cm^{3}$ (at $25^{\circ}$C
Vapor density = 1.3 (relative to air )
Molar Volume = 24470 $cm^{3}$/mol}
Thermodynamic Properties
Listed below are some of the thermodynamic properties of HCL:
Specific heat capacity cp (gas) = 0.7982 J/(gK)
Specific free energy of formation $\Delta_{f}G^{\circ}$ (gas) = -2.614 kJ/g
Specific heat of formation $\Delta_{f} H^{ \circ}$ (gas) = -2.532 kJ/g}
Specific entropy $S^{\circ}$ (gas) = 5.129 J/(gK)
Specific heat of vaporization = 0.414 \KJ/J
Specific heat of fusion = 0.05 KJ/g}
Critical Temperature = 325K
Critical Pressure = 8.27 MPa
Toxicity Property
Following are some of the toxicity properties of HCL:
Short term exposure limit = 7 mg/$m^{3}$
Ion Equivalent
The ion equivalence of HCL is given below:
$H^{+}$ (hydrogen cation) = 1
$Cl^{-}$(chloride anion) = 1
All Images/graphs are made using GeoGebra.
