JUMP TO TOPIC
Net Ionic Equation Calculator + Online Solver with Free Steps
A Net Ionic Equation Calculator is an online tool that shows all the properties, reactions, structures, and balanced equations of the input equation. This calculator provides details of each element and compound involved in the reaction.
A net ionic equation calculator is an accurate and quick tool that provides all the specifications of the compounds involved in the reaction. This makes it easy for the user to predict and identify the ionic compounds involved in the reaction and differentiate them from the spectator ions.
In a net ionic equation, only the species that are involved in the chemical reaction are displayed. This calculator makes it easy to identify these species. Moreover, the simple interface of the calculator makes it easy for the user to navigate the tool and obtain the answer.
What Is a Net Ionic Equation Calculator?
A Net Ionic Equation Calculator is an online calculator that is used to identify the iconic species involved in a chemical reaction.

Figure-1 Net Ionic Calculator
The net ionic equation calculator helps in this identification by providing specifications of each specie included in the reaction. Upon inserting the chemical equation, this calculator provides the balanced chemical equation, the names of the species, the structures of the species, the basic properties of the species, and their states in the chemical reaction.
Analyzing these properties makes it easier for the users to make predictions about the species that took part in the chemical reaction and separate them from the spectator ions. Take note that all the chemical species are analyzed in an aqueous state i.e, dissolved state.
The Net Ionic Equation Calculator is very easy to use and has a simple interface. Its interface consists of a prompt box that enables the user to enter the chemical equation for which they wish to obtain the net ionic equation.
The Net Ionic Equation Calculator is extremely accurate and provides the desired answer in a matter of seconds. It provides all the insights associated with the species, such as their melting and boiling points, their molar mass, their density, and much more. The Net Ionic Equation Calculator even provides information regarding the structures of the compound, which is one of its striking features.
How To Use the Net Ionic Equation Calculator
The Net Ionic Equation Calculator is extremely simple to use by following the steps mentioned below. Its user-friendly interface adds to the simplicity of this calculator.
All this calculator requires from the user is to enter any chemical equation.
The user can insert any chemical equation of their choice consisting of ionic compounds. Since the net ionic equations are devised for ions in an aqueous state, make sure to use chemical equations that include ionic compounds.
The interface of the net ionic calculator consists of one prompt box into which the user enters the chemical reaction. Listed below is a simple step-by-step guide that indicates how to use the net ionic equation calculator:
Step 1:
Analyze the chemical equation for which you want to draft the net ionic equation. Make sure the chemical equation consists of ionic compounds and also ensure that the chemical equation is balanced.
Step 2:
Enter this chemical equation into the prompt box. Again, ensure that it is balanced. If the chemical equation is not balanced, then the calculator will show an error and will not initiate the solution.
So make sure that the chemical equation is perfectly balanced.
Step 3:
After you have entered the chemical equation and have ensured the balance factor, the next thing you need to do is to click on the button that says “Submit.” This will initiate the solution.
Step 4:
Once you have clicked on the submit button, the calculator will take up to 5 seconds to display the answer. The calculator will display the answer in the form of multiple factors such as input interpretation, structure, basic properties, etc.
How Does The Net Ionic Equation Calculator Work?
The Net Ionic Equation Calculator works by specifying the properties of the given chemical equation. It is accurate, quick, and simple. It works by displaying all the specifications of the species included in the chemical reaction so the users can identify the active ions from the spectator ions.
The specifications displayed by the calculator include input interpretation, structure diagrams, basic properties, NFPA labels, enthalpy, rate constant, and much more.
These specifications enable the user to predict the net ionic equation.
What is a Net Ionic Equation?
A Net Ionic Equation is the equation that shows only the ionic species which were involved in the chemical reaction — it does not show the spectator ions.
The chemical equation that shows all the chemical species involved in the reaction is called a complete ionic equation. It also shows the spectator ions.
The difference between a complete ionic equation and a net ionic equation is that the net ionic equation does not show the spectator species whereas the complete ionic equation shows the spectator species.
Finding the Net Ionic Equation
The net ionic equation is very easy to find manually. First, choose any chemical equation that involves ionic compounds and write down its balanced chemical equation. This balanced chemical equation is the molar equation.
Next, transform the molar equation into a complete ionic equation by dissociating each ionic compound into its respective ions. Also, mention the phase of these species. The ions will all be in an aqueous phase after being dissolved.
Now, apply the rules of stability on both sides of the equation and cancel out the common species which are present on both sides of the equation. These canceled ions are the spectator ions and they do not take part in the chemical reaction.
After canceling out the common species, rewrite the leftover equation. This equation will only consist of the active ions which take part in the chemical reaction. This equation, which shows the ions taking part in the chemical reaction, is called a net ionic equation.
The Net Ionic Equation Calculator relieves you from the worries of calculating the net ionic equation of chemical reactions and makes the job easier for you.
Solved Examples
Below are some solved examples by using this calculator that will help you to develop a better understanding of the functionality of the net ionic equation calculator.
Example 1
Sulfuric acid $H_{2}SO_{4}$ is a strong acid that splits into $H^{+}$ and $SO_{4}^{2-}$ ions in the aqueous solution. When $H_{2}SO_{4}$ is combined with a strong base such as NaOH, the $Na^{+}$ and the $OH^{-}$ ions are disintegrated into the aqueous solution.
The two ionic compounds react with each other, which is indicated by the chemical equation shown below:
\[ H_{2}SO_{4(aq)} + 2NaOH_{(aq)} \rightarrow 2H_{2}O_{(l)} + Na_{2}SO_{4(aq)} \]
Identify the balanced net ionic equation of this chemical reaction.
Solution
To obtain the net ionic equation for this chemical equation, enter the equation in the prompt box of the net ionic equation calculator.
Upon entering the equation, some specifications will be displayed on the calculator:
Balanced Equation:
\[ H_{2}SO_{4(aq)} + 2NaOH_{(aq)} \rightarrow 2H_{2}O_{(l)} + Na_{2}SO_{4(aq)} \]
Word Equation:
\[ \text{sulfuric acid} + \text{sodium hydroxide} \rightarrow \text{water} + \text{sodium sulfate} \]
Equilibrium Constant:
\[ K_{c} = \frac{[H_{2}O]^{2} [Na_{2}SO_{4}]} {[H_{2}SO_{4}] [NaOH]^{2}} \]
Other specifications which are obtained include structures, thermodynamic properties (enthalpy), rate of reaction, chemical formulas, and substance properties which consist of density, molar mass, phase, surface tension, etc.
By analyzing these specifications, the following net ionic equation can be made:
\[ H^{+}_{(aq)} + OH^{-}_{(aq)} \rightarrow H_{2}O_{(l)} \]
This indicates that only the $H^{+}$ and $OH^{-}$ ions were the active ions in the chemical reaction and the rest were spectator ions.
Example 2
Aqueous solutions of $AgNO_{3}$ and NaCl were mixed in a container. Upon mixing, solid AgCl and $NaNO_{3}$ were formed. The following balanced chemical equation can be written for this reaction:
\[ AgNO_{3(aq)} + NaCl_{(aq)} \rightarrow AgCl_{(s)} + NaNO_{3(aq)} \]
Determine its net ionic equation.
Solution
Enter this equation into the prompt box of the calculator and click on the “Submit” button. After a few seconds, the calculator will display a variety of specifications of all the species involved in the chemical reaction.
Some of these specifications are given below:
Balanced Equation:
\[ AgNO_{3(aq)} + NaCl_{(aq)} \rightarrow AgCl_{(s)} + NaNO_{3(aq)} \]
Word Equation:
\[ \text{silver nitrate} + \text{sodium chloride} \rightarrow \text{silver chloride} + \text{sodium nitrate} \]
Equilibrium Constant:
\[ K_{c} = \frac {[AgCl] [NaNO_{3}]} {[AgNO_{3}] [NaCl] } \]
Besides these specifications, other factors which are displayed by the calculator include enthalpies, basic properties (density, molar mass, phase, odor, etc), chemical formulas, rate constant, and structures.
After analyzing these specifications, the following net ionic equation is obtained:
\[ Ag^{+}_{(aq)} + Cl^{-}_{(aq)} \rightarrow AgCl_{(s)} \]
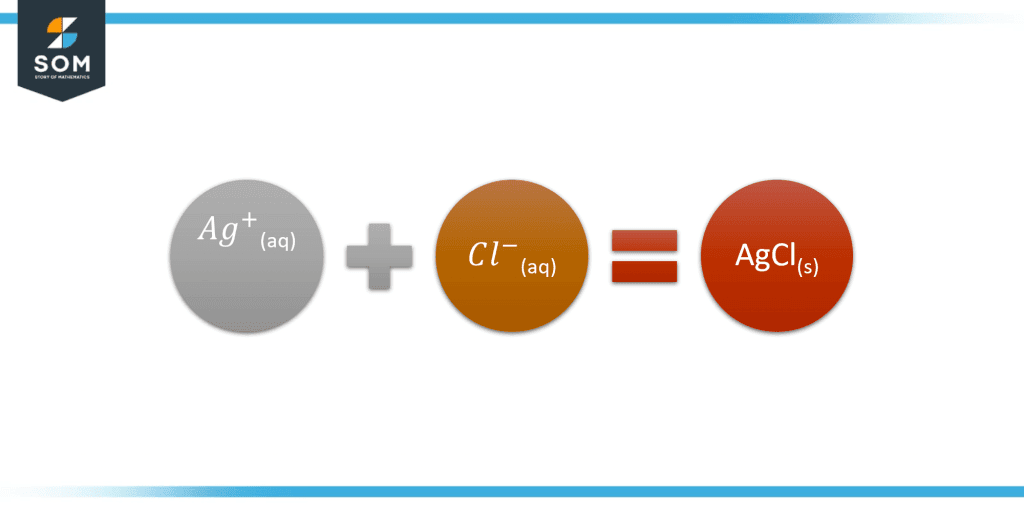
Figure-2 Solved Example of Ag and Cl
This indicates that the rest of the species involved in the reaction were spectator ions.
