JUMP TO TOPIC
Rate Constant Calculator + Online Solver With Free Steps
The Rate Constant Calculator is a tool used to calculate the rate constant k of any chemical equation. This tool is practical and convenient. It has been designed to determine the rate of reaction and constant k of the given chemical expression instantly and easily. The calculator layout includes the input tab for the rate of reaction, the molarity of reactant A, the order of reactant A, the molarity of reactant B, and the order of reactant B and computes the rate constant of reaction k as the output.
What Is the Rate Constant Calculator?
The Rate Constant Calculator is a calculator that is used to find the rate constant and concentration of the given substance given that rate laws are satisfied.It displays the results in both exact and decimal forms. The rate constant for the chemical reaction is a proportionality constant that depends on several factors such as temperature, catalyst, etc. The rate constant calculator has solved the problem of determining the rate constant. The calculated rate constants can be compared with the experimental values to cater to the errors. It is easy to use and handy, which makes it a perfect tool for experiments or solving complex homework tasks.How To Use the Rate Constant Calculator
The Rate Constant Calculator can be used to obtain the rate constant k of a chemical reaction by following a few simple steps mentioned below. All you need to do is figure out what you need to calculate and gather all the input data such as molarity of the reactants, order of the reactants, and order of the reaction so that you can easily find the value of the rate constant.In this section, you will discover how to use the rate constant calculator for determining the rate constant of any chemical reaction.Step 1:
First, analyze your query and determine the number of molecules of reactants reacting in the elementary step. Also, make sure that the chemical equation is balanced, otherwise you will get the wrong answer.Step 2:
Second, input the equation’s rate of reaction. In the “Equation’s rate of reaction,” specify the given rate of the reaction. The chemical reaction can be a zero-order, first-order, or second-order reaction depending on the chemical reaction.- If the order of the reaction is zero, this means that the rate of the reaction is equivalent to the rate constant of the reaction.
Reaction Rate = k
k = Reaction Rate
- If it is the first-order reaction, then the rate of reaction is equivalent to the product of the rate constant and the concentration.
Reaction Rate = k [A]
\[ k = \dfrac{Reaction\ Rate}{ [A] } \]Where [A] is the concentration of the reactant.- If the order of the reaction is second, then the rate of the reaction is equivalent to the product of rate constant and square of the concentration of reactant A. There can also be two distinct reactants such as A and B, so the reaction rate can be written as:
OR
Reaction Rate = k [A] [B]
\[ k = \dfrac { Reaction\ Rate }{ [A] [B] } \]Where [A] and [B] are the concentration of reactants A and B.Step 3:
Third, input the molarity or concentration of reactant A.Step 4:
In the next input tab, enter the order of the reactant A.Step 5:
If your reaction is a first-order reaction, then there must be only one reactant involved so you don’t have to enter the concentration or order of the reactant B.But if the chemical reaction is of second-order, then you have to input the concentration and order of reactant B. To do that, simply enter the molarity of reactant B.Step 6:
Now input the order of the reactant B.Step 7:
Once you have entered all the input values, press the submit button to see the results.Step 8:
The result of the rate constant k on this online calculator is expressed in both the exact and decimal forms. If you want to view the detailed step-by-step solution, just click the appropriate button shown on the screen and you can have the comprehensive solution.In conclusion, following these simple steps can help you use the calculator for any kind of chemical reaction.It is important to be noted that this calculator can only be used for the chemical reactions involving two distinct reagents, therefore for the reactions having more than two reactants, this online calculator cannot be used to obtain the value of the rate constant.How Does the Rate Constant Calculator Work?
The Rate Constant Calculator works by using the Reaction Rate formula and manipulating it to calculate the rate constant k of the chemical reaction.For instance, the rate of the first-order chemical reaction is given as:Rate = k [ Concentration of the reactant ]
Consider the following first-order reaction to determine the rate constant k:
Figure-1 First order Equation
What Is the Rate of Reaction?
The rate of reaction is the rate or speed at which any chemical reaction occurs. It determines the number of moles reacting per liter of the given solution in 1 second. The common units for the rate of reaction are M/sec, M/min, or $ mol/ sec * L $.The rate of the reaction can also be defined as the product of the rate constant and molar concentration of reactants where the Molar Concentration is given as:\[ Molar Concentration [M] = \dfrac{ Number\ of \ moles }{ Liters\ of \ solution } \]\[ M = \dfrac{ mol }{ L } \]What Is the Rate Constant of Reaction?
The rate constant k of the equation is the constant for any type of reaction given at some particular temperature. It can be calculated by using various methods and techniques. Some of them are mentioned below.Using Rate of Reaction Equation
It is the simplest technique mentioned above as well. You can simplify and modify the rate equation to determine the rate constant k. If you know the rate of reaction and concentration of the reactants in the chemical equation, then this method is the best one to calculate the value of the rate constant k.Using the Arrhenius Equation
The rate constant k depends on the temperature due to which Arrhenius Equation can also be used to determine the rate constant k.The Arrhenius equation is given as:\[ k = A\ exp ( \dfrac { -E }{ RT}) \]Where A is the concentration of the reactant and T is the temperature.Rate Constant of Reversible Reaction
For the reversible chemical reaction, there is a simple formula that can be used to determine the rate constant of the reaction.The formula is given as:\[ K = \dfrac{ k_2 }{ k_1 } \]Where K is known as the equilibrium constant of the chemical equation, and k1 and k2 are the rate constants of the forward and backward reaction, respectively.Therefore, using this equation, you can determine both the rate constants of the forward and the backward reactions.Finding the Rate Constant Of a Chemical Equation
The rate constant of the chemical equation can be found by following the steps mentioned below:- First, balance the given chemical equation so that both sides of the equation have an equal number of moles.
- Now, determine the order of the reaction for each compound or atom involved in the chemical reaction.
- Determine the initial concentration of all the reactants and raise them to the power of their specific order and multiply all of them together.
- Now, divide the rate of reaction and product of the concentrations of the reactants to determine the rate constant k.
Solved Examples
Here are some examples of how to determine the rate constant of different types of chemical equations.Example 1
Find the reaction rate constant k such that the initial concentration of the reactant A is 1M and the order of the reactant in the equation is 1. For the reactant B, the concentration of the reactant B is 2 M and the order of the reactant B is 1.Solution
Given that:Molar Concentration of reactant A = 1 M Order of the reactant A = 1 Molar Concentration of reactant B = 2 M Order of the reactant B = 1 The rate of the reaction = $ 1 \times 10^{-3} M/s $Input all these values in the calculator to get the results.The value of the rate constant k is given as: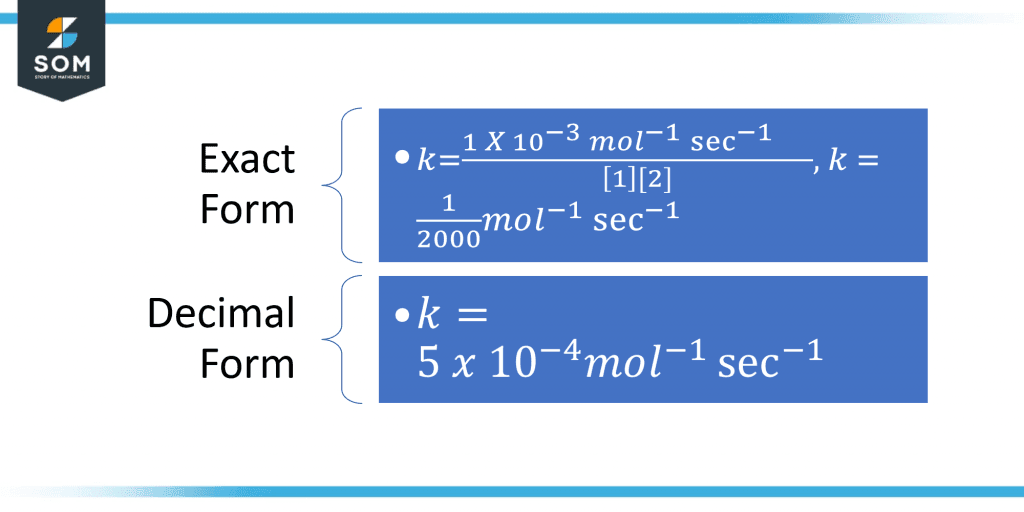
Figure-2 Solved Example
